
Interactions | Reactions | Processes
Classified as: Claisen Condensation
Claisen condensations proceed when an ester with an acidic alpha-hydrogen is reacted with base to form its conjugate base.
This nucleophilic carbanion then initiates the displacement of alkoxide ion. For more information look in the Chemogenesis webbook section on name reactions.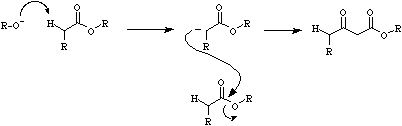
CH3COOR |
+ |
+ |
Base– Proton abstractor |
+ |
Na |
+ |
+ |
Na+ |
+ |
CH3CH2O– |
+ |
H2 |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com