
Interactions | Reactions | Processes
Classified as: Birch Reduction
Birch reduction involves an SET from the metal to the pi-system to give the radical anion. This species abstracts a proton from the solvent forming a radical. A second SET occurs giving the anion. This abstracts another proton from the solvent. Subtle changes in product can be obtained by controlling solvent pKa.
For more information look in the Chemogenesis webbook sections on redox chemistry and substitution, transfer, abstraction, displacement (STAD).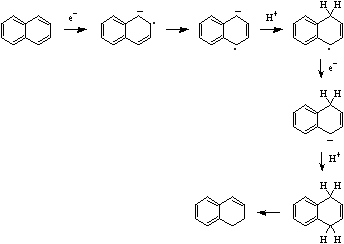
+ |
2 |
Na |
+ |
Hg |
+ |
2 |
CH3CH2OH |
+ |
2 |
Na+ |
+ |
2 |
CH3CH2O– |
+ |
2 |
Na |
+ |
+ |
4 |
[H] |
+ |
2 |
Na+ |
+ |
4 |
Na |
+ |
4 |
CH3CH2OH |
+ |
NH3 |
+ |
4 |
Na+ |
+ |
4 |
CH3CH2O– |
+ |
8 |
Li |
+ |
CH3CH2NH2 |
+ |
8 |
[H] |
+ |
8 |
Li+ |
+ |
2 |
Li |
+ |
2 |
CH3CH2NH2 |
+ |
2 |
Li+ |
+ |
2 |
+ |
2 |
Li |
+ |
CH3CH2NH2 |
+ |
2 |
[H] |
+ |
H2O |
+ |
CH3OH |
+ |
2 |
Li+ |
+ |
2 |
Li |
+ |
2 |
CH3CH2NH2 |
+ |
2 |
Li+ |
+ |
2 |
+ |
2 |
Li |
+ |
CH3CH2NH2 |
+ |
2 |
[H] |
+ |
H2O |
+ |
(CH3)2NH |
+ |
2 |
Li+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com