
Interactions | Reactions | Processes
Classified as: Dissolution: With Ionisation
Water dissolves ionic compounds by independently solvating the anions and cations. Ions are surrounded by a sphere of hydration.
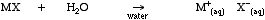
[H3O]+ |
+ |
[BF4]– |
HBF4(aq) |
+ |
H2O |
NaCl |
Na+ |
+ |
Cl– |
SO3 |
+ |
H2O |
H2SO4(aq) |
SO3 |
+ |
H2SO4(aq) |
H2SO4 |
CuO |
+ |
H2SO4(aq) |
CuSO4 |
+ |
H2O |
HBr |
+ |
CH3COOH |
HBr in CH3COOH 30% W/W |
NH3 |
+ |
H2O |
NH3(aq) |
CH3COOH |
+ |
H2O |
CH3COOH(aq) |
HCl |
+ |
H2O |
HCl(aq) |
H2S |
+ |
H2O |
H2S(aq) |
N2O4 |
[NO]+ |
+ |
[NO3]– |
SeO2 |
+ |
H2O |
H2SeO3 |
Na2[B4O5(OH)4] .8H2O |
+ |
5 |
H2O |
2 |
H3BO3 |
+ |
2 |
[B(OH)4]– |
+ |
2 |
Na+ |
+ |
8 |
H2O |
CuSO4.5H2O |
+ |
KNa Tartrate .4H2O |
+ |
NaOH |
+ |
H2O |
Fehling's Solution |
Cu |
+ |
HOOH |
+ |
2 |
HCl(conc) |
Cu2+ |
+ |
2 |
Cl– |
+ |
2 |
H2O |
[Al(H2O)6]3+ |
+ |
4 |
NaOH(aq) |
[Al(OH)4]– |
+ |
4 |
Na+ |
+ |
6 |
H2O |
Al(H2O)3(OH)3 Al(OH)3 |
+ |
NaOH(aq) |
[Al(OH)4]– |
+ |
Na+ |
+ |
3 |
H2O |
Al(H2O)3(OH)3 Al(OH)3 |
+ |
3 |
H+ |
[Al(H2O)6]3+ |
Fe(H2O)4(OH)2 |
+ |
2 |
H+ |
[Fe(H2O)6]2+ |
Co(H2O)4(OH)2 |
+ |
2 |
H+ |
[Co(H2O)6]2+ |
Cu(H2O)4(OH)2 |
+ |
2 |
H+ |
[Cu(H2O)6]2+ |
Fe(H2O)3(OH)3 |
+ |
3 |
H+ |
[Fe(H2O)6]3+ |
Cr(H2O)3(OH)3 |
+ |
3 |
H+ |
[Cr(H2O)6]2+ |
AgBr |
+ |
2 |
+ |
hν |
[Ag(S2O3)2]3– |
+ |
Br– |
AgCl |
+ |
2 |
NH3(aq) |
[Ag(NH3)2]+ |
+ |
Cl– |
Al(OH)3 |
+ |
NaOH |
[Al(OH)4]– |
+ |
Na+ |
2 |
Al(OH)3 |
+ |
3 |
H2SO4(aq) |
Al2(SO4)3 |
+ |
6 |
H2O |
2 |
Al(OH)3 |
+ |
3 |
H2SO4(aq) |
2 |
Al3+ |
+ |
3 |
[SO4]2– |
+ |
6 |
H2O |
CuCl2 |
+ |
2 |
HCl(conc) |
[CuCl4]2– |
+ |
2 |
H+ |
MgO |
+ |
2 |
HNO3(aq) |
Mg2+ |
+ |
2 |
[NO3]– |
+ |
H2O |
MgO |
+ |
2 |
H+ |
+ |
H2O |
Mg2+ |
+ |
H2O |
SiO2 |
+ |
2 |
NaOH |
+ |
H2O |
Na2SiO3 solution |
+ |
H2O |
SiO2 |
+ |
2 |
[OH]– |
[SiO3]2– |
+ |
H2O |
AgCl |
+ |
HCl(conc) |
[AgCl2]– |
+ |
H+ |
FeSO4.7H2O |
+ |
H2O |
[Fe(H2O)6]2+ |
+ |
[SO4]2– |
+ |
H2O |
Mg(OH)2 |
+ |
H2O |
Mg2+ |
+ |
2 |
[OH]– |
Ca(OH)2 |
+ |
H2O |
Ca(OH)2(aq) |
Ca(OH)2 |
+ |
H2O |
Ca2+ |
+ |
2 |
[OH]– |
Sr(OH)2 |
+ |
H2O |
Sr2+ |
+ |
2 |
[OH]– |
Ba(OH)2 |
+ |
H2O |
Ba2+ |
+ |
2 |
[OH]– |
Be(OH)2 |
+ |
2 |
H+ |
+ |
H2O |
Be2+ |
+ |
2 |
H2O |
Be(OH)2 |
+ |
2 |
HCl(aq) |
Be2+ |
+ |
2 |
Cl– |
+ |
2 |
H2O |
Be(OH)2 |
+ |
2 |
[OH]– |
+ |
H2O |
[Be(OH)4]2– |
Be(OH)2 |
+ |
2 |
NaOH(aq) |
2 |
Na+ |
+ |
[Be(OH)4]2– |
Mg(OH)2 |
+ |
2 |
H+ |
+ |
H2O |
Mg2+ |
+ |
2 |
H2O |
Mg(OH)2 |
+ |
2 |
HCl(aq) |
Mg2+ |
+ |
2 |
Cl– |
+ |
2 |
H2O |
BaCl2 |
+ |
2 |
NaOH(aq) |
+ |
H2O |
Ba(OH)2 |
+ |
2 |
NaCl |
BeCl2 |
+ |
4 |
NaOH(aq) |
+ |
H2O |
[Be(OH)4]2– |
+ |
4 |
Na+ |
+ |
2 |
Cl– |
BaCl2 |
+ |
2 |
NaOH(aq) |
+ |
H2O |
Ba2+ |
+ |
2 |
Na+ |
+ |
2 |
Cl– |
+ |
2 |
[OH]– |
K2CO3 |
+ |
2 |
HCl(aq) |
2 |
K+ |
+ |
2 |
Cl– |
+ |
H2O |
+ |
CO2 |
AgF |
+ |
H2O |
Ag+ |
+ |
F– |
AgBr |
+ |
2 |
.880 NH3 |
[Ag(NH3)2]+ |
+ |
Br– |
Al2O3.2H2O (+Fe, Ti oxides) |
Al2O3 |
AgF |
+ |
NH3(aq) |
Ag+ |
+ |
F– |
+ |
NH3(aq) |
AgBr |
+ |
NH3(aq) |
Ag+ |
+ |
Br– |
HCl |
+ |
H2O |
HCl(conc) |
Au |
+ |
Aqua Regia |
+ |
Cl– |
[AuCl4]– |
+ |
NO |
+ |
2 |
H2O |
NaCl |
+ |
H2O |
Brine |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com