
Interactions | Reactions | Processes
Classified as: Tautomerism
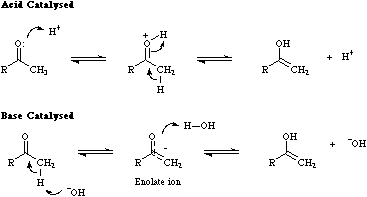
Tautomerism occurs when two distinct functional group types are in equilibrium with each other. Equilibrium position is strongly influenced by the precise structure of the functions, solvent, temperature etc. and is often catalysed by acid and/or base. The classic example of tautomerism is the "keto-enol" system in which ketones with an alpha-hydrogen are in equilibrium with the corresponding enol. Many reaction of such ketones proceed via their enol form. These reaction pathways are not available to (say) diaryl ketones which are devoid of enolisable functions.
+ |
Br2 |
+ |
CH3COOH |
+ |
HBr |
HC≡CH |
+ |
H2O |
+ |
H+ |
CH3CHO |
+ |
hν |
H2C=CH2 |
+ |
2 |
+ |
NaOH(aq) |
+ |
H2O |
+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com