
Interactions | Reactions | Processes
Classified as: Reduction: Of Substrate By Reagent
A substrate is deemed to be reduced by a reducing agent if the reducing agent (1) adds electrons to the substrate by single electron transfer or SET, (2) adds electron rich hydrogen (as H2, or H-) to the substrate, (3) adds electron density from the substrate by reversing polarisation by removing electronegative oxygen or (4) electronegative halogen atoms.
For more information look in the Chemogenesis webbook section on redox chemistry.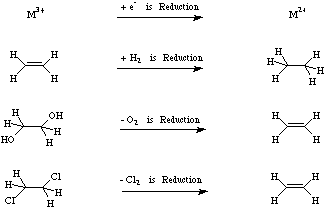
(CH3CO)2CO |
+ |
LiAlH4 |
+ |
8 |
[H] |
+ |
2 |
CH3CH2OH |
+ |
H2O |
RCOCl |
+ |
LiAlH4 |
+ |
RCH2OH |
RCOCl |
+ |
Li[Al(OtBu)3H] 1.0M in THF |
+ |
RCHO |
RCOCl |
+ |
H2 |
+ |
Pd on BaSO4 |
RCHO |
+ |
HCl |
RCHO |
+ |
NaBH4 |
RCH2OH |
RCHO |
+ |
H2N–NH2 |
+ |
OH–(aq) |
R–CH3 |
+ |
N2 |
RCHO |
+ |
Zn(Hg) |
+ |
H+(aq) |
R–CH3 |
+ |
Zn2+ |
+ |
LiAlH4 |
+ |
RCH2NH2 |
RCOOH |
+ |
LiAlH4 |
RCH2OH |
2 |
RCOOH |
+ |
B2H6 |
2 |
RCH2OH |
+ |
LiAlH4 |
RCH2OH |
+ |
R–OH |
+ |
RCHO |
+ |
R–OH |
+ |
NaBH4 |
R2CHOH |
+ |
H2N–NH2 |
+ |
OH–(aq) |
+ |
N2 |
R–CN |
+ |
LiAlH4 |
+ |
4 |
[H] |
RCH2NH2 |
R–CN |
+ |
RCHO |
+ |
2 |
Fe |
+ |
6 |
H+ |
+ |
2 |
H2O |
+ |
2 |
Fe3+ |
+ |
3 |
Sn |
+ |
6 |
H+ |
+ |
3 |
Sn2+ |
+ |
2 |
H2O |
+ |
NaBH4 |
+ |
2 |
[H] |
R–NO2 |
+ |
2 |
(CH3CH2O)3P |
R–N |
+ |
2 |
(CH3CH2O)3P=O |
+ |
LiAlH4 |
+ |
2 |
[H] |
CH3CH2OH |
HCHO |
+ |
H– |
[CH3–O]– |
RCHO |
+ |
H– |
R–CH2O– |
CH3CHO |
+ |
H– |
CH3CH2O– |
+ |
H– |
[(CH3)2CH–O]– |
+ |
H2 |
+ |
Pt |
+ |
Na |
+ |
Na+ |
HOOH |
+ |
Fe2+ |
[H2O2]+• |
+ |
Fe3+ |
+ |
Na |
+ |
Na+ |
2 |
C |
+ |
O2 |
2 |
CO |
12 |
+ |
4 |
NaBH4 |
+ |
4 |
4 |
+ |
3 |
NaBF4 |
+ |
4 |
3 |
+ |
+ |
+ |
3 |
+ |
(CH3)2S→BH3 |
+ |
(CH3)2S |
CuSO4 |
+ |
Zn |
+ |
H+(aq) |
Cu |
+ |
Zn2+ |
+ |
[SO4]2– |
R–C≡C–R |
+ |
H2 |
+ |
Pd on C |
R–C≡C–R |
+ |
2 |
H2 |
+ |
Pd on C |
R–C≡C–R |
+ |
2 |
H2 |
+ |
Pt on carbon |
+ |
H2 |
+ |
Pd on C |
+ |
H2 |
+ |
Rh on Carbon |
RCOOH |
+ |
2 |
H2 |
+ |
Ru on carbon |
RCH2OH |
+ |
2 |
H2O |
RCHO |
+ |
H2 |
+ |
Ru on carbon |
+ |
H2O |
RCH2OH |
+ |
H2 |
+ |
Pd on C |
+ |
2 |
H2 |
+ |
Pd on C |
+ |
H2O |
+ |
H2 |
+ |
Ru on carbon |
+ |
3 |
H2 |
+ |
Rh on Carbon |
2 |
+ |
6 |
H2 |
+ |
Pd on C |
+ |
NH3 |
+ |
2 |
H2 |
+ |
Pd on C |
+ |
H+(aq) |
+ |
R–NH2 |
+ |
3 |
H2 |
+ |
Rh on Carbon |
+ |
H2 |
+ |
Pd on C |
+ |
RCl |
+ |
H2 |
+ |
Pd on C |
R–H |
+ |
HCl |
RBr |
+ |
H2 |
+ |
Pd on C |
R–H |
+ |
HBr |
R–I |
+ |
H2 |
+ |
Pd on C |
R–H |
+ |
HI |
+ |
H2 |
+ |
Pd on C |
R–CH2CH2OH |
RHC=N–NH2 |
+ |
H2 |
+ |
Pt on carbon |
R–CH2–NNH2 |
+ |
H2 |
+ |
Pt on carbon |
+ |
H2 |
+ |
Ru on carbon |
+ |
H2O |
R2CHOH |
+ |
H2 |
+ |
Pd on C |
+ |
4 |
H2 |
+ |
Rh on Carbon |
R–CN |
+ |
2 |
H2 |
+ |
Pd on C |
RCH2NH2 |
2 |
R–CN |
+ |
4 |
H2 |
+ |
Rh on Carbon |
(RCH2)2NH |
+ |
NH3 |
R–CN |
+ |
R–NH2 |
+ |
4 |
H2 |
+ |
Rh on Carbon |
+ |
NH3 |
3 |
R–CN |
+ |
6 |
H2 |
+ |
Pd on C |
2 |
(RCH2)3N |
+ |
2 |
NH3 |
+ |
2 |
H2 |
+ |
Pd on C |
2 |
+ |
4 |
H2 |
+ |
Pt on carbon |
+ |
NH3 |
+ |
H2 |
+ |
H2O |
+ |
Pd on C |
+ |
H+(aq) |
+ |
NH3 |
+ |
3 |
H2 |
+ |
Ni |
+ |
2 |
H2O |
+ |
2 |
H2 |
+ |
Pt on carbon |
+ |
CH3CH2OH |
+ |
H2O |
+ |
2 |
H2 |
+ |
Pt on carbon |
+ |
H2O |
R2N–NO |
+ |
2 |
H2 |
+ |
Pd on C |
(R)2N–NH2 |
+ |
H2O |
+ |
2 |
H2 |
+ |
Pd on C |
+ |
H2O |
+ |
3 |
H2 |
+ |
Rh on Carbon |
+ |
H2 |
+ |
Pd on C |
+ |
H2O |
+ |
+ |
H2 |
+ |
Pt on carbon |
+ |
H2O |
+ |
2 |
H2 |
+ |
Pd on C |
R–CN |
+ |
2 |
+ |
Pd on C |
RCH2NH2 |
+ |
2 |
2 |
+ |
+ |
Pd on C |
2 |
+ |
RCHO |
+ |
LiAlH4 |
+ |
RCH2OH |
+ |
LiAlH4 |
+ |
R2CHOH |
R–NO2 |
+ |
LiAlH4 |
R–NH2 |
RCOO– |
+ |
LiAlH4 |
RCH2OH |
+ |
H2 |
+ |
Pd on C |
+ |
CH3OH |
+ |
+ |
H2 |
+ |
Pd on C |
+ |
(CH3)3COH |
+ |
+ |
H2 |
+ |
Pd on C |
+ |
+ |
+ |
H2 |
+ |
Pd on C |
+ |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
NaBH4 |
+ |
3 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
NaBH4 |
+ |
3 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
NaBH4 |
+ |
4 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
+ |
2 |
Na |
+ |
Hg |
+ |
2 |
CH3CH2OH |
+ |
2 |
Na+ |
+ |
2 |
CH3CH2O– |
+ |
2 |
Na |
+ |
+ |
4 |
[H] |
+ |
2 |
Na+ |
+ |
4 |
Na |
+ |
4 |
CH3CH2OH |
+ |
NH3 |
+ |
4 |
Na+ |
+ |
4 |
CH3CH2O– |
+ |
8 |
Li |
+ |
CH3CH2NH2 |
+ |
8 |
[H] |
+ |
8 |
Li+ |
+ |
2 |
Li |
+ |
2 |
CH3CH2NH2 |
+ |
2 |
Li+ |
+ |
2 |
+ |
2 |
Li |
+ |
CH3CH2NH2 |
+ |
2 |
[H] |
+ |
H2O |
+ |
CH3OH |
+ |
2 |
Li+ |
+ |
2 |
Li |
+ |
2 |
CH3CH2NH2 |
+ |
2 |
Li+ |
+ |
2 |
+ |
2 |
Li |
+ |
CH3CH2NH2 |
+ |
2 |
[H] |
+ |
H2O |
+ |
(CH3)2NH |
+ |
2 |
Li+ |
CaO |
+ |
3 |
C |
CaC2 |
+ |
CO |
+ |
H– |
RCHO |
+ |
2 |
R3COH |
+ |
2 |
K |
2 |
+ |
2 |
K+ |
+ |
H2 |
+ |
H2 |
+ |
Pd on C |
+ |
H2 |
+ |
Pd on C |
+ |
H2 |
+ |
Ni/H2 Raney Nickel |
R–CH3 |
+ |
H2 |
+ |
Pt on carbon |
3 |
+ |
Al(OiPr)3 |
3 |
+ |
3 |
SiO2 |
+ |
C |
Si |
+ |
CO2 |
Fe2O3 |
+ |
3 |
C |
2 |
Fe |
+ |
3 |
CO |
ZnO |
+ |
C |
Zn |
+ |
CO |
MgO |
+ |
C |
Mg |
+ |
CO |
PbO |
+ |
C |
+ |
CO |
3 |
Mn3O4 |
+ |
8 |
Al |
9 |
Mn |
+ |
4 |
Al2O3 |
Cr2O3 |
+ |
2 |
Al |
2 |
Cr |
+ |
Al2O3 |
B2O3 |
+ |
2 |
Al |
2 |
B |
+ |
Al2O3 |
TiCl4 |
+ |
2 |
Mg |
+ |
Ar |
Ti |
+ |
2 |
MgCl2 |
TiCl4 |
+ |
4 |
Na |
+ |
Ar |
Ti |
+ |
4 |
NaCl |
B(OCH3)3 |
+ |
4 |
NaH |
NaBH4 |
+ |
3 |
NaOCH3 |
AlCl3 |
+ |
4 |
LiH |
LiAlH4 |
+ |
3 |
LiCl |
2 |
H2 |
+ |
Al |
+ |
Li |
LiAlH4 |
SiCl4 |
+ |
LiAlH4 |
SiH4 |
+ |
LiCl |
+ |
AlCl3 |
2 |
BF3 |
+ |
6 |
NaH |
B2H6 |
+ |
6 |
NaF |
B2O3 |
+ |
2 |
Al |
+ |
3 |
H2 |
+ |
AlCl3 |
B2H6 |
+ |
Al2O3 |
4 |
+ |
3 |
NaBH4 |
+ |
2 |
B2H6 |
+ |
3 |
NaBF4 |
+ |
4 |
SO2 |
+ |
2 |
H2S |
3 |
S |
+ |
2 |
H2O |
3 |
Cl2O |
+ |
10 |
NH3 |
6 |
NH4Cl |
+ |
2 |
N2 |
+ |
3 |
H2O |
2 |
CH3OH |
+ |
2 |
Na |
2 |
NaOCH3 |
+ |
H2 |
2 |
CO |
+ |
NaH |
HCOONa |
+ |
C |
2 |
NH3 |
+ |
2 |
e–(am) |
2 |
[H2N]– |
+ |
H2 |
2 |
[NH4]+ |
+ |
2 |
e–(am) |
2 |
NH3 |
+ |
H2 |
2 |
NaNO3 |
+ |
C |
2 |
NaNO2 |
+ |
CO2 |
NaNO3 |
+ |
Zn |
NaNO2 |
+ |
ZnO |
PCl3 |
+ |
LiAlH4 |
+ |
3 |
[H] |
PH3 |
+ |
3 |
Cl– |
2 |
BCl3 |
+ |
LiAlH4 |
+ |
6 |
[H] |
B2H6 |
+ |
6 |
Cl– |
Na2SO4 |
+ |
2 |
C |
Na2S |
+ |
2 |
CO2 |
HC≡CH |
+ |
2 |
Na |
+ |
NH3 |
NaC≡CNa |
+ |
H2 |
HC≡CH |
+ |
2 |
K |
+ |
NH3 |
KC≡CK |
+ |
H2 |
HC≡CH |
+ |
2 |
Rb |
+ |
NH3 |
RbC≡CRb |
+ |
H2 |
HC≡CH |
+ |
2 |
Cs |
+ |
NH3 |
CsC≡CCs |
+ |
H2 |
BeSO4 |
+ |
4 |
C |
BeS |
+ |
4 |
CO |
MgSO4 |
+ |
4 |
C |
MgS |
+ |
4 |
CO |
CaSO4 |
+ |
4 |
C |
CaS |
+ |
4 |
CO |
SrSO4 |
+ |
4 |
C |
SrS |
+ |
4 |
CO |
BaSO4 |
+ |
4 |
C |
BaS |
+ |
4 |
CO |
2 |
BeO |
+ |
3 |
C |
Be2C |
+ |
2 |
CO |
MgO |
+ |
3 |
C |
MgC2 |
+ |
CO |
SrO |
+ |
3 |
C |
SrC2 |
+ |
CO |
BaO |
+ |
3 |
C |
BaC2 |
+ |
CO |
2 |
BCl3 |
+ |
3 |
H2 |
2 |
B |
+ |
6 |
HCl |
B2O3 |
+ |
3 |
Mg |
2 |
B |
+ |
3 |
MgO |
SiO2 |
+ |
3 |
C |
SiC |
+ |
2 |
CO |
SiO2 |
+ |
2 |
C |
Si |
+ |
2 |
CO |
SiCl4 |
+ |
2 |
Mg |
Si |
+ |
2 |
MgCl2 |
Na2[SiF6] |
+ |
4 |
Na |
Si |
+ |
6 |
NaF |
GeO2 |
+ |
2 |
H2 |
Ge |
+ |
2 |
H2O |
C60 |
+ |
3 |
K |
K3C60 |
PdCl2 |
+ |
CO |
+ |
H2O |
Pd |
+ |
CO2 |
+ |
2 |
HCl |
Fe2O3 |
+ |
3 |
CO |
2 |
Fe |
+ |
3 |
CO2 |
CuO |
+ |
CO |
Cu |
+ |
CO2 |
SiCl4 |
+ |
4 |
LiH |
SiH4 |
+ |
4 |
LiCl |
GeCl4 |
+ |
LiAlH4 |
+ |
GeH4 |
+ |
LiCl |
+ |
AlCl3 |
GeO2 |
+ |
NaBH4 |
+ |
H2O |
GeH4 |
+ |
NaBO2 Na3[B3O6] |
SnCl4 |
+ |
LiAlH4 |
+ |
SnH4 |
+ |
LiCl |
+ |
AlCl3 |
Sb2S3 |
+ |
3 |
Fe |
2 |
Sb |
+ |
3 |
FeS |
2 |
P2O5 |
+ |
10 |
C |
P |
+ |
10 |
CO |
Bi2O3 |
+ |
3 |
C |
2 |
Bi |
+ |
3 |
CO |
2 |
CuSO4 |
+ |
H2N–NH2 |
2 |
Cu |
+ |
N2 |
+ |
2 |
H2SO4 |
H2N–NH2 |
+ |
Zn |
+ |
2 |
HCl |
2 |
NH3 |
+ |
ZnCl2 |
3 |
HN3 |
+ |
2 |
Na |
2 |
NaN3 |
+ |
NH3 |
+ |
N2 |
2 |
HNO2 |
+ |
2 |
I– |
+ |
2 |
H+ |
2 |
NO |
+ |
I2 |
+ |
2 |
H2O |
NO2 |
+ |
CO |
NO |
+ |
CO2 |
N2O4 |
+ |
2 |
CO |
2 |
NO |
+ |
2 |
CO2 |
SO3 |
+ |
H2S |
+ |
2 |
AgF2 |
+ |
H2 |
2 |
AgF |
+ |
2 |
HF |
Te(OH)6 |
+ |
6 |
HI |
TeI4 |
+ |
I2 |
+ |
6 |
H2O |
[IO3]– |
+ |
5 |
I– |
+ |
6 |
H+ |
3 |
I2 |
+ |
3 |
H2O |
Cl2 |
+ |
H2O |
HClO |
+ |
HCl |
F2 |
+ |
H2 |
2 |
HF |
Cl2 |
+ |
H2 |
2 |
HCl |
Br2 |
+ |
H2 |
+ |
Pt |
2 |
HBr |
TiO2 |
+ |
2 |
C |
+ |
2 |
Cl2 |
TiCl4 |
+ |
2 |
CO |
2 |
ClO2 |
+ |
HOOH |
+ |
2 |
NaOH |
2 |
NaClO2 |
+ |
O2 |
+ |
2 |
H2O |
XeF2 |
+ |
H2 |
Xe |
+ |
2 |
HF |
XeF4 |
+ |
2 |
H2 |
Xe |
+ |
4 |
HF |
XeF6 |
+ |
3 |
H2 |
Xe |
+ |
6 |
HF |
NO2 |
+ |
CO |
NO |
+ |
CO2 |
S4N4 |
+ |
4 |
Ag |
+ |
2 |
Ag2S |
+ |
N2 |
RCOCl |
+ |
RCHO |
+ |
+ |
N2 |
+ |
R2S |
+ |
H2O |
+ |
H2N–NH2 |
+ |
CH3CH2OH |
+ |
N2 |
+ |
H2O |
2 |
(CH3)3COH |
+ |
2 |
K |
2 |
+ |
H2 |
+ |
H2 |
+ |
Pd on C |
+ |
H2 |
+ |
CH4 |
4 |
H2O |
+ |
3 |
FeCl2 |
H2 |
+ |
Fe3O4 |
+ |
6 |
HCl |
+ |
H2 |
2 |
Ca3(PO4)2 |
+ |
10 |
Coke |
P |
+ |
6 |
CaO |
+ |
10 |
CO |
4 |
CaSO4 |
+ |
2 |
C |
4 |
CaO |
+ |
4 |
SO2 |
+ |
2 |
CO2 |
2 |
SO2 |
+ |
Zn |
ZnS2O3 |
2 |
SO2 |
+ |
HCOONa |
+ |
NaOH |
+ |
CO2 |
+ |
H2O |
8 |
SO2 |
+ |
NaBH4 |
+ |
8 |
NaOH |
4 |
+ |
NaBO2 Na3[B3O6] |
+ |
6 |
H2O |
S |
+ |
H2 |
H2S |
I2 |
+ |
SO2 |
+ |
2 |
H2O |
2 |
HI |
+ |
H2SO4 |
R–NO2 |
+ |
3 |
H2 |
+ |
Pd on C |
R–NH2 |
+ |
2 |
H2O |
+ |
H2 |
+ |
Pd on C |
+ |
R–OH |
+ |
2 |
H2 |
+ |
Pd on C |
RCH2OH |
+ |
R–OH |
+ |
2 |
H2 |
+ |
Pd on C |
+ |
H2O |
+ |
LiAlH4 |
R–CH2CH2OH |
+ |
LiAlH4 |
RCOOH |
+ |
RCH2OH |
+ |
+ |
R2CHOH |
R–CN |
+ |
RCH2NH2 |
+ |
+ |
R–CH2CH2OH |
+ |
RCH2OH |
+ |
R–OH |
KCl |
+ |
Na |
K |
+ |
NaCl |
2 |
KF |
+ |
CaC2 |
2 |
K |
+ |
CaF2 |
+ |
2 |
C |
BeF2 |
+ |
Mg |
Be |
+ |
MgF2 |
3 |
CaO |
+ |
2 |
Al |
3 |
Ca |
+ |
Al2O3 |
2 |
Na2Cr2O7 .H2O |
+ |
3 |
C |
2 |
Cr2O3 |
+ |
2 |
Na2CO3 |
+ |
CO2 |
+ |
4 |
H2O |
Na2Cr2O7 .H2O |
+ |
S |
Cr2O3 |
+ |
Na2SO4 |
+ |
2 |
H2O |
Na2Cr2O7 .H2O |
+ |
2 |
NH4Cl |
Cr2O3 |
+ |
2 |
NaCl |
+ |
N2 |
+ |
6 |
H2O |
Cr2O3 |
+ |
3 |
C |
2 |
Cr |
+ |
3 |
CO |
2 |
B2O3 |
+ |
6 |
Mg |
+ |
C |
B4C |
+ |
6 |
MgO |
2 |
B2O3 |
+ |
4 |
Al |
+ |
C |
B4C |
+ |
2 |
Al2O3 |
TiO2 |
+ |
3 |
C |
TiC |
+ |
2 |
CO |
HC≡CH |
+ |
H2 |
H2C=CH2 |
+ |
H2 |
+ |
H2 |
+ |
H2 |
CH3CHO |
+ |
H2O |
+ |
H2 |
+ |
H2 |
+ |
2 |
H2 |
+ |
3 |
H2 |
+ |
Pd on C |
+ |
H2 |
+ |
H2O |
+ |
+ |
+ |
H2 |
+ |
H2O |
[VO2]+ |
+ |
Zn |
+ |
2 |
H+ |
+ |
4 |
H2O |
[VO]2+ |
+ |
Zn2+ |
[VO]2+ |
+ |
Zn |
+ |
2 |
HCl(aq) |
[VCl2(H2O)4]+ |
+ |
Zn2+ |
+ |
2 |
H2O |
[VO]2+ |
+ |
Zn |
+ |
H2SO4(aq) |
[V(H2O)6]3+ |
+ |
Zn2+ |
+ |
[SO4]2– |
[VCl2(H2O)4]+ |
+ |
Zn |
+ |
2 |
H2O |
[V(H2O)6]2+ |
+ |
Zn2+ |
+ |
2 |
Cl– |
[V(H2O)6]3+ |
+ |
Zn |
[V(H2O)6]2+ |
+ |
Zn2+ |
[Cr2O7]2– |
+ |
3 |
Zn |
+ |
14 |
H+ |
2 |
Cr3+ |
+ |
3 |
Zn2+ |
+ |
7 |
H2O |
[Cr2O7]2– |
+ |
3 |
Zn |
+ |
14 |
HCl(aq) |
+ |
H2O |
2 |
[CrCl2(H2O)4]+ |
+ |
3 |
Zn2+ |
+ |
10 |
Cl– |
[CrCl2(H2O)4]+ |
+ |
Zn |
+ |
2 |
H2O |
[Cr(H2O)6]2+ |
+ |
Zn2+ |
+ |
2 |
Cl– |
2 |
[MnO4]– |
+ |
5 |
Zn |
+ |
16 |
H+ |
+ |
4 |
H2O |
2 |
[Mn(H2O)6]2+ |
+ |
5 |
Zn2+ |
2 |
[MnO4]– |
+ |
5 |
Zn |
+ |
16 |
HCl(aq) |
+ |
4 |
H2O |
2 |
[Mn(H2O)6]2+ |
+ |
5 |
Zn2+ |
+ |
16 |
Cl– |
+ |
2 |
I– |
+ |
Fe2+ |
2 |
[SO4]2– |
+ |
I2 |
+ |
2 |
Fe2+ |
2 |
[SO4]2– |
+ |
2 |
Fe3+ |
2 |
Fe3+ |
+ |
2 |
I– |
2 |
Fe2+ |
+ |
I2 |
2 |
[MnO4]– |
+ |
5 |
H2SO3 |
2 |
Mg2+ |
+ |
5 |
[SO4]2– |
+ |
3 |
H2O |
+ |
4 |
H+ |
[CuCl4]2– |
+ |
Cu |
[CuCl4]3– |
+ |
Cu+ |
2 |
[VO2]+ |
+ |
3 |
Zn |
+ |
8 |
H+ |
+ |
8 |
H2O |
2 |
[V(H2O)6]2+ |
+ |
3 |
Zn2+ |
2 |
[Ag(CN)2]– |
+ |
Zn |
2 |
Ag |
+ |
Zn2+ |
+ |
4 |
[CN]– |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
H2O |
+ |
4 |
H2 |
+ |
Ni |
+ |
LiAlH4 |
+ |
8 |
[H] |
+ |
3 |
Sn |
+ |
6 |
HCl(conc) |
+ |
3 |
Sn2+ |
+ |
6 |
Cl– |
+ |
2 |
H2O |
+ |
3 |
H2 |
+ |
Ni |
+ |
2 |
H2O |
Fe2O3 |
+ |
2 |
Al |
2 |
Fe |
+ |
Al2O3 |
[ClO]– |
+ |
2 |
I– |
+ |
2 |
H+ |
Cl– |
+ |
I2 |
+ |
H2O |
TiO2 |
+ |
2 |
C |
+ |
2 |
Cl2 |
TiCl4 |
+ |
2 |
CO |
2 |
Fe2O3 |
+ |
3 |
C |
4 |
Fe |
+ |
3 |
CO2 |
[SO4]2– |
+ |
2 |
Br– |
+ |
4 |
H+ |
SO2 |
+ |
Br2 |
+ |
2 |
H2O |
H2SO4 |
+ |
2 |
Br– |
+ |
2 |
H+ |
SO2 |
+ |
Br2 |
+ |
2 |
H2O |
FeTiO3 |
+ |
2 |
C |
+ |
2 |
Cl2 |
TiCl4 |
+ |
2 |
CO |
+ |
FeO |
2 |
AgNO3 |
+ |
Zn |
+ |
H2O |
2 |
Ag |
+ |
Zn(NO3)2 |
2 |
Ag+ |
+ |
Zn |
+ |
H2O |
2 |
Ag |
+ |
Zn2+ |
PbO2 |
+ |
2 |
Cl– |
+ |
4 |
H+ |
Pb2+ |
+ |
Cl2 |
+ |
2 |
H2O |
2 |
NH4NO3 |
+ |
C |
2 |
N2 |
+ |
CO2 |
+ |
4 |
H2O |
HOOH |
+ |
2 |
I– |
+ |
2 |
H+ |
2 |
H2O |
+ |
I2 |
2 |
Ag+ |
+ |
SO2 |
+ |
2 |
H2O |
2 |
Ag |
+ |
[SO4]2– |
+ |
4 |
H+ |
FeS |
+ |
Mg |
MgS |
+ |
Fe |
2 |
NO |
+ |
2 |
CO |
+ |
Catalytic Converter Pt | Rh | Pd | Ir |
N2 |
+ |
2 |
CO2 |
+ |
H2 |
+ |
Ni |
+ |
H2 |
+ |
Ni |
+ |
H– |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
LiAlH4 |
+ |
2 |
[H] |
+ |
H– |
+ |
NaBH4 |
+ |
2 |
[H] |
+ |
LiAlH4 |
+ |
2 |
[H] |
CH3CN |
+ |
2 |
H2 |
+ |
Ni |
CH3CH2NH2 |
+ |
H2 |
+ |
Ni |
+ |
LiAlH4 |
+ |
6 |
[H] |
Fe2O3 |
+ |
3 |
H2 |
2 |
Fe |
+ |
3 |
H2O |
2 |
Cu2+ |
+ |
4 |
I– |
+ |
H2O |
2 |
CuI |
+ |
I2 |
+ |
2 |
H2 |
+ |
Ni |
[MnO4]– |
+ |
3 |
Fe2+ |
+ |
4 |
H+ |
MnO2 |
+ |
3 |
Fe3+ |
+ |
2 |
H2O |
[Cr2O7]2– |
+ |
6 |
V2+ |
+ |
14 |
H+ |
2 |
Cr3+ |
+ |
6 |
V3+ |
+ |
7 |
H2O |
2 |
Cr3+ |
+ |
Zn |
+ |
H2O |
2 |
Cr2+ |
+ |
Zn2+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com