
Interactions | Reactions | Processes
Classified as: Hydroformylation
Hydroformylation, or the "oxo reaction", involves adding carbon monoxide and hydrogen to an alkene to give an extended carbon chain with an aldehyde terminus.
Linear aldehydes are generated over branched aldehydes. The reaction requires high pressure (200 - 450 bar) and so is more common as in industrial method than as a lab technique. The cobalt (or Ru) carbonyl hydride catalyst adds across the alkene double bond (or the alkene inserts into the Co-H bond), to form an intermediate with rearranges to a second intermediate which is reduced to aldehyde plus regenerated catalyst.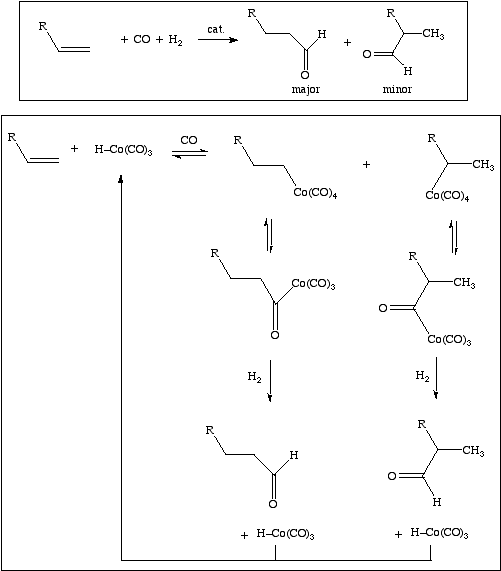
Co2(CO)8 |
+ |
H2 |
2 |
HCo(CO)4 |
H2C=CH2 |
+ |
HCo(CO)3 |
+ |
CO |
+ |
H2 |
+ |
HCo(CO)3 |
HCo(CO)4 |
HCo(CO)3 |
+ |
CO |
H2C=CH2 |
+ |
CO + H2 Oxo Gas |
+ |
Co |
CH3–CH=CH2 |
+ |
CO + H2 Oxo Gas |
+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com