
Interactions | Reactions | Processes
Classified as: Substitution: Nucleophilic First Order
First order nucleophilic substitution occurs when a nucleofugal Lewis base leaving group spontaneously leaves a carbon centre (often with solvent assistance) to form a planar carbenium ion which subsequently attacked and complexed by a nucleophilic Lewis base.
For more information look in the Chemogenesis webbook sections on substitution reactions and substitution, transfer, abstraction, displacement (STAD).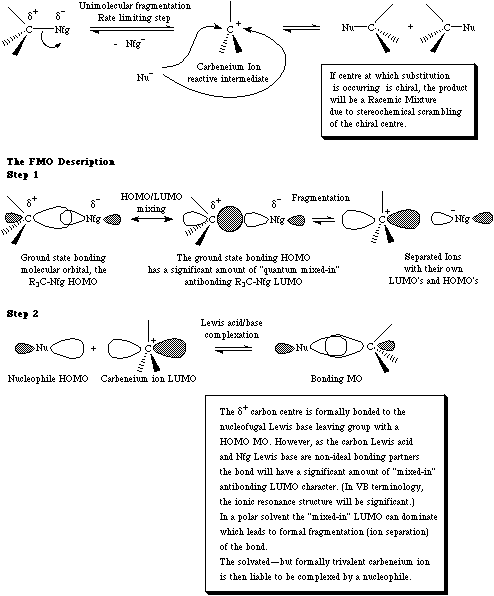
(CH3)3COH |
+ |
HCl(conc) |
+ |
H2O |
(CH3)3COH |
+ |
HBr |
(CH3)3CBr |
+ |
H2O |
(CH3)3COH |
+ |
HI |
(CH3)3CI |
+ |
H2O |
+ |
[OH]– |
+ |
+ |
Br– |
+ |
CH3COOH |
+ |
+ |
HCl |
+ |
[NO2]– |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com