
Interactions | Reactions | Processes
Classified as: Cycle of Copper Reactions
Copper can be dissolved in nitric acid to form the copper(II) nitrate solution, heated with hydroxide to form the copper(II) hydroxide and then the oxide. The oxide can be taken up in dilute sulfuric acid to form the sulfate which can be reduced to metallic copper with zinc and HCl.
This is an excellent practical for beginning chemistry students.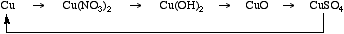
Cu |
+ |
4 |
HNO3 5 molar |
Cu(NO3)2 |
+ |
2 |
NO2 |
+ |
2 |
H2O |
Cu(NO3)2 |
+ |
2 |
NaOH |
+ |
H2O |
Cu(OH)2 |
+ |
2 |
NaNO3 |
Cu(OH)2 |
+ |
OH–(aq) |
CuO |
+ |
H2O |
CuO |
+ |
H2SO4(aq) |
CuSO4 |
+ |
H2O |
CuSO4 |
+ |
Zn |
+ |
H+(aq) |
Cu |
+ |
Zn2+ |
+ |
[SO4]2– |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com