
Interactions | Reactions | Processes
Classified as: Ambidentate Behaviour: Selectivity of beta-propiolactone
According to Pearsons hard soft [Lewis] acid base principle, hard Lewis acids have a strong affinity for hard Lewis bases and soft Lewis acids have a strong affinity for soft Lewis bases.
beta-Propiolactone reacts with hard nucleophiles (such as HO-) by acylation, and soft nucleophiles by alkylation. Note that while hard vs soft comparisons can be made for carefully selected pairs of species and/or reactive centres, the HSAB analysis is NOT general. For more information look in the Chemogenesis webbook section on Pearson's HSAB principle.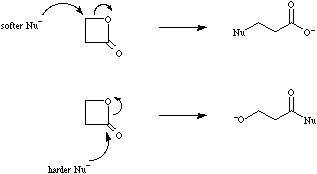
+ |
RO– |
+ |
[CN]– |
+ |
[R–S]– |
+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com