
Interactions | Reactions | Processes
Classified as: Elimination vs Substitution
Subtle alterations to molecular geometry can dramatically alter the course of a particular reaction. E2 mechanisms require that the Nfg and Efg be diaxial for elimination to occur.
If for steric reasons a diaxial conformation cannot be achieved there remains the possibility of Sn2 substitution. During elimination the Lewis base reagent is acting as a proton abstracting Brønsted base, in the substitution it is acting as a nucleophile. For more information look in the Chemogenesis webbook section on competing mechanistic pathways.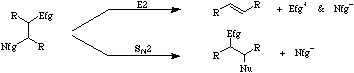
+ |
[OH]– |
+ |
(CH3)3N |
+ |
[OH]– |
+ |
(CH3)3N |
+ |
H2O |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com