
Interactions | Reactions | Processes
Classified as: van der Waals Attraction
There are three types of van der Waals Attraction:
Dipole/Dipole attraction in which molecules with permanent dipole moments interact with each other so that the dipoles line up "end-to-end" and which at temperatures below the materials melting point show long range order and crystal structure. Dipole/Induced-Dipole Attraction: Molecular dipoles are able to induce weak dipoles in adjacent non-polar species. London Force Attraction: The very fact that it is possible to liquefy helium and the other Group VIII gases demonstrates that there must be some type of inter-atomic attraction taking place, known as the "London force". For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.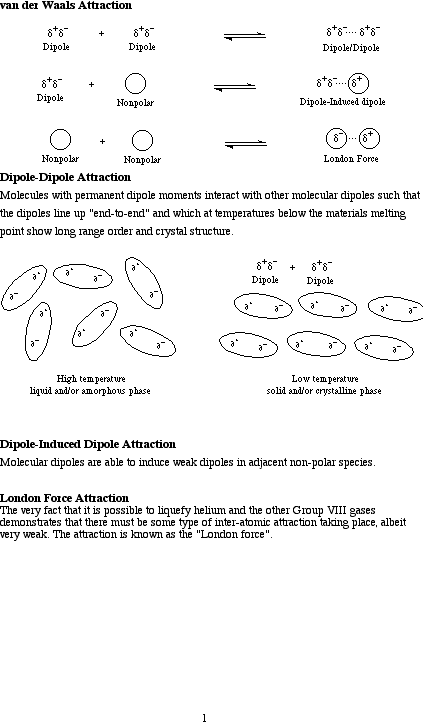
CH3I |
+ |
CH3I |
CH3I |
+ |
Ar |
Ar |
+ |
Ar |
2 |
Ar(l) |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com