
Interactions | Reactions | Processes
Classified as: Anion Bridge Bonding
Bridging bonds occur when a mono-valent hydride or halogen anion is held between a pair of Lobe-LUMO Lewis acid centres.
The classic example of the bridging bond is found in diborane, B2H6. Bridging bonds are most commonly found in simple inorganic hydrides and halides, such as PdCl2. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.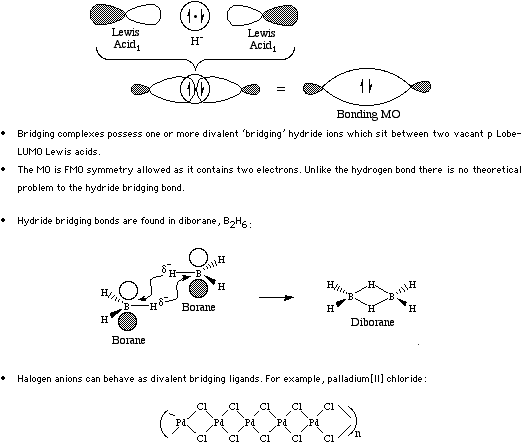
2 |
BH3 |
B2H6 |
Pd2+ |
+ |
2 |
Cl– |
PdCl2 |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com