
Interactions | Reactions | Processes
Classified as: Hydrogen Bond Complexation
Hydrogen bonding occurs when a proton is attracted to, and held between, a pair Lewis bases.
The small low mass H+ ion quantum tunnels between the two bases generating a time averaged three centre bonded state. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.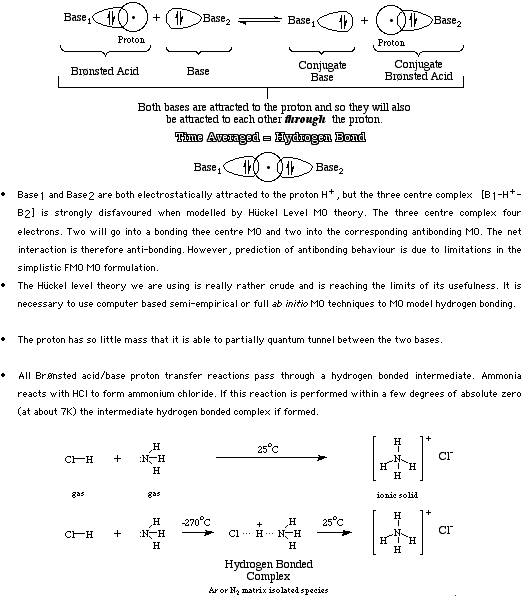
+ |
H2O |
+ |
HCl |
+ |
NH3 |
HF |
+ |
HF |
HF |
+ |
F– |
[FHF]– |
H2O |
+ |
H2O |
2 |
CH3OH |
NH3 |
+ |
H2O |
CF4 |
+ |
H2O |
CH3CONH2 |
+ |
CH3COOH |
+ |
CH3COOH |
CH3OH |
+ |
HF |
+ |
Cytosine C |
+ |
Guanine G |
C≡G |
Thymine T |
+ |
Adenine A |
T=A |
[H3O]+ |
+ |
H2O |
[H3O]+ |
+ |
3 |
H2O |
KF |
+ |
HF |
K[HF2] |
6 |
HF |
(HF)6 |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com