
Interactions | Reactions | Processes
Classified as: Type 11 Lewis Acid/Base Complexation Chemistry
Generally Onium ion Lewis acids do not complex with Lobe-HOMO Lewis bases, because the Onium ion is liable to transfer a ligand to the Lobe-HOMO Lewis base.
NH4OH (ammonium hydroxide) is clearly a Type 11 Lewis acid/base complex. This species exists in an equilibrium state with NH3 + H2O. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.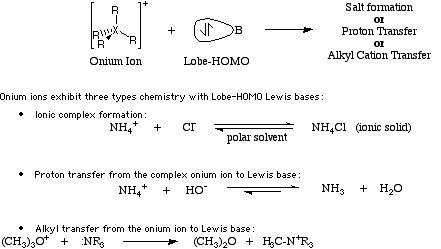
+ |
HBF4(aq) |
+ |
H2O |
+ |
+ |
[BF4]– |
[H3O]+ |
+ |
+ |
Cl– |
[NH4]+ |
+ |
[CH3COO]– |
[NH4]+[CH3CO2]– |
[NH4]+ |
+ |
Cl– |
NH4Cl |
[NH4]+ |
+ |
[NO3]– |
NH4NO3 |
2 |
[NH4]+ |
+ |
S2– |
(NH4)2S |
[NH4]+ |
+ |
[HS]– |
NH4HS |
+ |
I– |
+ |
+ |
+ |
[NH4]+ |
+ |
[FHF]– |
[NH4]+ [FHF]– |
NH3 |
+ |
HCl |
NH4Cl |
[NH4]+ |
+ |
[ClO4]– |
NH4ClO4 |
2 |
[NH4]+ |
+ |
(NH4)2S2O8 |
2 |
[NH4]+ |
+ |
[SO3]2– |
+ |
H2O |
(NH4)2SO3 .H2O |
2 |
[NH4]+ |
+ |
(NH4)2S2O3 |
[NH4]+ |
+ |
F– |
NH4F |
[NH4]+ |
+ |
[VO3]– |
NH4VO3 |
[H3O]+ |
+ |
[OH]– |
2 |
H2O |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com