
Interactions | Reactions | Processes
Classified as: Type 10 Lewis Acid/Base Complexation Chemistry
Onium Ion Lewis acids form classic charge controlled (highly ionic) complexes with Complex anion Lewis bases. An example of such a complex is ammonium tetrafluoroborate.
Type 10 complexes are soluble in polar solvents in which the ions are fully dissociated. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.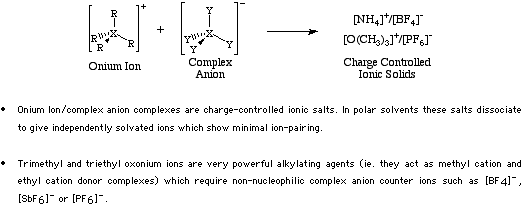
[R4N]+ |
+ |
[BF4]– |
[R4N]+ [BF4]– |
[H3O]+ |
+ |
[BF4]– |
HBF4(aq) |
+ |
H2O |
+ |
B2H6 |
+ |
2 |
NH3 |
[H3N–BH2–NH3]+ [BH4]– |
[NH4]+ |
+ |
[ClO4]– |
NH4ClO4 |
BF3 |
+ |
ClF3 |
[ClF2]+ |
+ |
[BF4]– |
SbF5 |
+ |
ClF3 |
[ClF2]+ |
+ |
[SbF6]– |
I2 |
+ |
I–Cl |
+ |
AlCl3 |
[I3]+ |
+ |
[AlCl4]– |
Cl2 |
+ |
ClF3 |
+ |
AsF5 |
[Cl3]+ |
+ |
[AsF6]– |
+ |
F2 |
Br2 |
+ |
BrF3 |
+ |
AsF5 |
[Br3]+ |
+ |
[AsF6]– |
+ |
F2 |
XeF2 |
+ |
AsF5 |
[XeF]+ |
+ |
[AsF6]– |
XeF2 |
+ |
PF5 |
[XeF]+ |
+ |
[PF6]– |
XeF2 |
+ |
SbF5 |
[XeF]+ |
+ |
[SbF6]– |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com