
Interactions | Reactions | Processes
Classified as: Type 7 Lewis Acid/Base Complexation Chemistry
Type 7 complexes, which form when s-LUMO Lewis acids complex with Lobe-HOMO Lewis bases, are commonly used as the basic reagents of organic and inorganic chemistry: methyl lithium, sodamide, potassium hydroxide, sodium fluoride, potassium hydrogen phthalate, etc.
Type 7 complexes are generally ionic or highly polar covalent species (ie they are charge controlled complexes) and are soluble in polar solvents. The proton abstracting power of a particular Type 7 reagent depends upon the pKa of its conjugate Brønsted acid as proton abstraction power is only subtly altered by the s-LUMO Lewis acid counter ion. Lithium Type 7 complexes are less ionic and less basic than the corresponding sodium salts. Li+ < Na+ < K+ < Cs+ For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.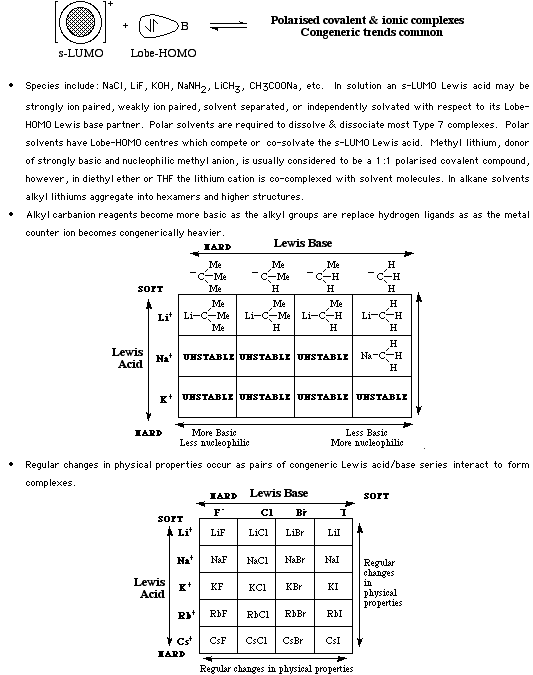
+ |
KMnO4 |
+ |
+ |
[MnO4]– |
Na+ |
+ |
[H2N]– |
NaNH2 |
Na+ |
+ |
Cl– |
NaCl |
Na+ |
+ |
[OH]– |
NaOH |
Li+ |
+ |
[CH3]– |
LiCH3 |
Li+ |
+ |
K+ |
+ |
[OH]– |
KOH |
K+ |
+ |
K+ |
+ |
[(CH3)3C–O]– |
Na+ |
+ |
[N3]– |
NaN3 |
Na+ |
+ |
[HSO3]– |
NaHSO3 |
Na+ |
+ |
[CN]– |
NaCN |
Na+ |
+ |
I– |
NaI |
Mg2+ |
+ |
R– |
+ |
X– Spectator Anion |
RMgX |
Ba2+ |
+ |
[CO3]2– |
BaCO3 |
Ba2+ |
+ |
2 |
[OH]– |
Ba(OH)2 |
Be2+ |
+ |
2 |
Br– |
BeBr2 |
2 |
Cs+ |
+ |
[CO3]2– |
Cs2CO3 |
Ca2+ |
+ |
2 |
[CH3COO]– |
Ca(CH3COO)2 |
Ca2+ |
+ |
S2– |
CaS |
Ca2+ |
+ |
O2– |
CaO |
3 |
Li+ |
+ |
N3– |
Li3N |
K+ |
+ |
Na+ |
+ |
[NO2]– |
NaNO2 |
Mg2+ |
+ |
R– |
+ |
Cl– |
RMgCl |
Mg2+ |
+ |
R– |
+ |
Br– |
RMgBr |
Mg2+ |
+ |
[CH3]– |
+ |
I– |
CH3MgI |
Li+ |
+ |
R– |
RLi |
Li+ |
+ |
Br– |
LiBr |
Li+ |
+ |
Li+ |
+ |
HC≡C– |
R–C≡C–Li |
Li+ |
+ |
Li+ |
+ |
Ba2+ |
+ |
[C2O4]2– |
BaC2O4 |
Ba2+ |
+ |
[CrO4]2– |
BaCrO4 |
Ba2+ |
+ |
[SO4]2– |
BaSO4 |
Ca2+ |
+ |
[CO3]2– |
CaCO3 |
Ca2+ |
+ |
[C2O4]2– |
CaC2O4 |
Ca2+ |
+ |
2 |
F– |
CaF2 |
Ca2+ |
+ |
[SO4]2– |
CaSO4 |
Mg2+ |
+ |
[CO3]2– |
MgCO3 |
Mg2+ |
+ |
[C2O4]2– |
+ |
2 |
H2O |
MgC2O4 .H2O |
Mg2+ |
+ |
2 |
F– |
MgF2 |
Mg2+ |
+ |
2 |
[OH]– |
Mg(OH)2 |
Sr2+ |
+ |
[CO3]2– |
SrCO3 |
Sr2+ |
+ |
[C2O4]2– |
+ |
H2O |
SrC2O4 |
Sr2+ |
+ |
[SO4]2– |
SrSO4 |
2 |
Na+ |
+ |
[SO3]2– |
+ |
7 |
H2O |
Na2SO3 .7H2O |
2 |
Na+ |
+ |
[SO3]2– |
Na2SO3 |
2 |
Na+ |
+ |
S2– |
+ |
9 |
H2O |
Na2S.9H2O |
2 |
Na+ |
+ |
S2– |
Na2S |
Cu2+ |
+ |
[CO3]2– |
CuCO3 (unknown) |
2 |
Na+ |
+ |
[CO3]2– |
Na2CO3 |
Na+ |
+ |
Br– |
NaBr |
K+ |
+ |
I– |
KI |
K+ |
+ |
Br– |
KBr |
K+ |
+ |
F– |
KF |
Mg2+ |
+ |
O2– |
MgO |
Ca2+ |
+ |
2 |
Br– |
CaBr2 |
Ca2+ |
+ |
2 |
Cl– |
CaCl2 |
Ca2+ |
+ |
[WO4]2– |
CaWO4 |
2 |
Na+ |
+ |
[SO4]2– |
Na2SO4 |
Mg2+ |
+ |
[SO4]2– |
+ |
7 |
H2O |
MgSO4 .7H2O |
Ca2+ |
+ |
[SO4]2– |
+ |
2 |
H2O |
CaSO4 .2H2O |
2 |
Na+ |
+ |
[B4O7]2– |
+ |
10 |
H2O |
Na2[B4O5(OH)4] .8H2O |
Na+ |
+ |
[NO3]– |
NaNO3 |
K+ |
+ |
Cl– |
KCl |
+ |
Ca2+ |
+ |
–C≡C– |
CaC2 |
2 |
Li+ |
+ |
O2– |
Li2O |
2 |
Na+ |
+ |
O2– |
Na2O |
2 |
Rb+ |
+ |
O2– |
Rb2O |
2 |
Cs+ |
+ |
O2– |
Cs2O |
2 |
Li+ |
+ |
–O–O– |
Li2O2 |
2 |
Na+ |
+ |
–O–O– |
Na2O2 |
K+ |
+ |
[O–O]– |
K2O |
Li+ |
+ |
[O–O]– |
LiO2 |
Na+ |
+ |
[O–O]– |
NaO2 |
2 |
K+ |
+ |
O2– |
KO2 |
2 |
K+ |
+ |
–O–O– |
K2O2 |
2 |
Rb+ |
+ |
–O–O– |
Rb2O2 |
Rb+ |
+ |
[O–O]– |
RbO2 |
2 |
Cs+ |
+ |
–O–O– |
Cs2O2 |
Cs+ |
+ |
[O–O]– |
CsO2 |
2 |
K+ |
+ |
[CO3]2– |
K2CO3 |
K+ |
+ |
[HCO3]– |
KHCO3 |
Na+ |
+ |
[HCO3]– |
NaHCO3 |
Cs+ |
+ |
[OH]– |
CsOH |
Rb+ |
+ |
[OH]– |
RbOH |
2 |
Na+ |
+ |
Na2S2O3 |
2 |
Li+ |
+ |
–C≡C– |
LiC≡CLi |
K+ |
+ |
3 |
+ |
H+ |
Li+ |
+ |
+ |
H+ |
Li+ |
+ |
2 |
+ |
H+ |
Na2CO3 |
+ |
Ca(OH)2 |
CaCO3 |
+ |
2 |
NaOH |
Be2+ |
+ |
O2– |
BeO |
Sr2+ |
+ |
O2– |
SrO |
Ba2+ |
+ |
O2– |
BaO |
Ba2+ |
+ |
–O–O– |
BaO2 |
Be2+ |
+ |
2 |
[OH]– |
Be(OH)2 |
Ca2+ |
+ |
2 |
[OH]– |
Ca(OH)2 |
Sr2+ |
+ |
2 |
[OH]– |
Sr(OH)2 |
BeO |
+ |
H2O |
Be(OH)2 |
MgO |
+ |
H2O |
Mg(OH)2 |
CaO |
+ |
H2O |
Ca(OH)2 |
SrO |
+ |
H2O |
Sr(OH)2 |
BaO |
+ |
H2O |
Ba(OH)2 |
BaO2 |
+ |
2 |
H+ |
Ba2+ |
+ |
HOOH |
Ca2+ |
+ |
2 |
[OH]– |
+ |
CO2 |
+ |
H2O |
CaCO3 |
+ |
H2O |
CaCO3 |
+ |
CO2 |
+ |
H2O |
+ |
H2O |
Ca2+ |
+ |
2 |
[HCO3]– |
Be2+ |
+ |
[CO3]2– |
BeCO3 |
3 |
Li+ |
+ |
P3– |
Li3P |
3 |
Li+ |
+ |
As3– |
Li3As |
3 |
Li+ |
+ |
Sb3– |
Li3Sb |
3 |
Na+ |
+ |
P3– |
Na3P |
3 |
Na+ |
+ |
As3– |
Na3As |
3 |
Na+ |
+ |
Sb3– |
Na3Sb |
3 |
K+ |
+ |
P3– |
K3P |
3 |
K+ |
+ |
As3– |
K3As |
3 |
K+ |
+ |
Sb3– |
K3Sb |
3 |
Rb+ |
+ |
P3– |
Rb3P |
3 |
Rb+ |
+ |
As3– |
Rb3As |
3 |
Rb+ |
+ |
Sb3– |
Rb3Sb |
3 |
Cs+ |
+ |
P3– |
Cs3P |
3 |
Cs+ |
+ |
As3– |
Cs3As |
3 |
Cs+ |
+ |
Sb3– |
Cs3Sb |
Li+ |
+ |
F– |
LiF |
Li+ |
+ |
Cl– |
LiCl |
Li+ |
+ |
I– |
LiI |
Na+ |
+ |
F– |
NaF |
Rb+ |
+ |
F– |
RbF |
Rb+ |
+ |
Cl– |
RbCl |
Rb+ |
+ |
Br– |
RbBr |
Rb+ |
+ |
I– |
RbI |
Cs+ |
+ |
F– |
CsF |
Cs+ |
+ |
Cl– |
CsCl |
Cs+ |
+ |
Br– |
CsBr |
Cs+ |
+ |
I– |
CsI |
2 |
Li+ |
+ |
S2– |
Li2S |
2 |
K+ |
+ |
S2– |
K2S |
2 |
Rb+ |
+ |
S2– |
Rb2S |
2 |
Cs+ |
+ |
S2– |
Cs2S |
Li+ |
+ |
[HS]– |
LiSH |
Na+ |
+ |
[HS]– |
NaSH |
K+ |
+ |
[HS]– |
KSH |
Rb+ |
+ |
[HS]– |
RbSH |
Cs+ |
+ |
[HS]– |
CsSH |
LiOH |
+ |
H2S |
LiSH |
+ |
H2O |
NaOH |
+ |
H2S |
NaSH |
+ |
H2O |
KOH |
+ |
H2S |
KSH |
+ |
H2O |
RbOH |
+ |
H2S |
RbSH |
+ |
H2O |
CsOH |
+ |
H2S |
CsSH |
+ |
H2O |
2 |
LiOH |
+ |
H2S |
Li2S |
+ |
2 |
H2O |
2 |
KOH |
+ |
H2S |
K2S |
+ |
2 |
H2O |
2 |
RbOH |
+ |
H2S |
Rb2S |
+ |
2 |
H2O |
2 |
CsOH |
+ |
H2S |
Cs2S |
+ |
2 |
H2O |
Li2S |
+ |
H2O |
LiSH |
+ |
LiOH |
Na2S |
+ |
H2O |
NaSH |
+ |
NaOH |
K2S |
+ |
H2O |
KSH |
+ |
KOH |
Rb2S |
+ |
H2O |
RbSH |
+ |
RbOH |
Cs2S |
+ |
H2O |
CsSH |
+ |
CsOH |
2 |
Li+ |
+ |
Se2– |
Li2Se |
2 |
Na+ |
+ |
Se2– |
Na2Se |
2 |
K+ |
+ |
Se2– |
K2Se |
2 |
Rb+ |
+ |
Se2– |
Rb2Se |
2 |
Cs+ |
+ |
Se2– |
Cs2Se |
2 |
Li+ |
+ |
Te2– |
Li2Te |
2 |
Na+ |
+ |
Te2– |
Na2Te |
2 |
K+ |
+ |
Te2– |
K2Te |
2 |
Rb+ |
+ |
Te2– |
Rb2Te |
2 |
Cs+ |
+ |
Te2– |
Cs2Te |
2 |
LiNH2 |
+ |
NH3 |
LiNH2 |
+ |
NH3 |
Li+ |
+ |
[H2N]– |
LiNH2 |
K+ |
+ |
[H2N]– |
KNH2 |
Rb+ |
+ |
[H2N]– |
RbNH2 |
Cs+ |
+ |
[H2N]– |
CsNH2 |
2 |
Li+ |
+ |
[HN]2– |
LiNH2 |
2 |
Na+ |
+ |
–C≡C– |
NaC≡CNa |
2 |
K+ |
+ |
–C≡C– |
KC≡CK |
2 |
Rb+ |
+ |
–C≡C– |
RbC≡CRb |
2 |
Cs+ |
+ |
–C≡C– |
CsC≡CCs |
3 |
Be2+ |
+ |
2 |
N3– |
Be3N2 |
3 |
Mg2+ |
+ |
2 |
N3– |
Mg3N2 |
3 |
Ca2+ |
+ |
2 |
N3– |
Ca3N2 |
3 |
Sr2+ |
+ |
2 |
N3– |
Sr3N2 |
3 |
Ba2+ |
+ |
2 |
N3– |
Ba3N2 |
3 |
Be2+ |
+ |
2 |
P3– |
Be3P2 |
3 |
Mg2+ |
+ |
2 |
P3– |
Mg3P2 |
3 |
Ca2+ |
+ |
2 |
P3– |
Ca3P2 |
3 |
Sr2+ |
+ |
2 |
P3– |
Sr3P2 |
3 |
Ba2+ |
+ |
2 |
P3– |
Ba3P2 |
Be2+ |
+ |
S2– |
BeS |
Sr2+ |
+ |
S2– |
SrS |
Ba2+ |
+ |
S2– |
BaS |
Be2+ |
+ |
Se2– |
BeS |
Mg2+ |
+ |
Se2– |
MgSe |
Ca2+ |
+ |
Se2– |
CaSe |
Sr2+ |
+ |
Se2– |
SrSe |
Ba2+ |
+ |
Se2– |
BaSe |
Be2+ |
+ |
Te2– |
BeTe |
Mg2+ |
+ |
Te2– |
MgTe |
Ca2+ |
+ |
Te2– |
CaTe |
Sr2+ |
+ |
Te2– |
SrTe |
Ba2+ |
+ |
Te2– |
BaTe |
Be2+ |
+ |
2 |
F– |
BeF2 |
Be2+ |
+ |
2 |
Cl– |
BeCl2 |
Be2+ |
+ |
2 |
I– |
BeI2 |
Mg2+ |
+ |
2 |
Cl– |
MgCl2 |
Mg2+ |
+ |
2 |
Br– |
MgBr2 |
Mg2+ |
+ |
2 |
I– |
MgI2 |
Ca2+ |
+ |
2 |
I– |
CaI2 |
Sr2+ |
+ |
2 |
F– |
SrF2 |
Sr2+ |
+ |
2 |
Cl– |
SrCl2 |
Sr2+ |
+ |
2 |
Br– |
SrBr2 |
Sr2+ |
+ |
2 |
I– |
SrI2 |
Ba2+ |
+ |
2 |
F– |
BaF2 |
Ba2+ |
+ |
2 |
Cl– |
BaCl2 |
Ba2+ |
+ |
2 |
Br– |
BaBr2 |
Ba2+ |
+ |
2 |
I– |
BaI2 |
Be2+ |
+ |
2 |
[H2N]– |
Be(NH2)2 |
Mg2+ |
+ |
2 |
[H2N]– |
Mg(NH2)2 |
Ca2+ |
+ |
2 |
[H2N]– |
Ca(NH2)2 |
Sr2+ |
+ |
2 |
[H2N]– |
Sr(NH2)2 |
Ba2+ |
+ |
2 |
[H2N]– |
Ba(NH2)2 |
Be2+ |
+ |
[SO4]2– |
BeSO4 |
Mg2+ |
+ |
[SO4]2– |
MgSO4 |
Mg2+ |
+ |
–C≡C– |
MgC2 |
Sr2+ |
+ |
–C≡C– |
SrC2 |
Ba2+ |
+ |
–C≡C– |
BaC2 |
2 |
Be2+ |
+ |
C4– |
Be2C |
Tl+ |
+ |
[I3]– |
TlI3 |
Na+ |
+ |
[BO2]– [B3O6]3– |
NaBO2 Na3[B3O6] |
Be2+ |
+ |
[SiO3]2– |
Be2SiO4 |
3 |
Ca2+ |
+ |
2 |
[PO4]3– |
Ca3(PO4)2 |
Ca2+ |
+ |
[SiO3]2– |
CaSiO3 |
K+ |
+ |
[O3]– |
KO3 |
2 |
Na+ |
+ |
Na2S4O6 |
2 |
Na+ |
+ |
2 |
Na+ |
+ |
2 |
Na+ |
+ |
KF |
+ |
HF |
K[HF2] |
K+ |
+ |
[FHF]– |
K[HF2] |
Na+ |
+ |
[ClO3]– |
NaClO3 |
Na+ |
+ |
[ClO2]– |
NaClO2 |
K+ |
+ |
[ClO3]– |
KClO3 |
K+ |
+ |
[ClO4]– |
KClO4 |
Na+ |
+ |
[ClO4]– |
NaClO4 |
K+ |
+ |
[BrO4]– |
NaBrO4 |
K+ |
+ |
[BrO3]– |
NaBrO3 |
I–Cl |
+ |
KCl |
K+ |
+ |
[ICl2]– |
I–Cl |
+ |
KI |
K+ |
+ |
[I2Cl]– |
Ca2+ |
+ |
2 |
[HCO3]– |
Ca(HCO3)2 |
Ca(HCO3)2 |
+ |
H2SO4 |
CaSO4 |
+ |
2 |
CO2 |
+ |
2 |
H2O |
Na+ |
+ |
Ca2+ |
+ |
2 |
+ |
Ca2+ |
+ |
2 |
Na+ |
2 |
H+ |
+ |
K+ |
+ |
[NO3]– |
2 |
KNO3 |
2 |
Na+ |
+ |
[C2O4]2– |
K+ |
+ |
[CN]– |
KCN |
+ |
+ |
2 |
K+ |
+ |
[Cr2O7]2– |
K2Cr2O7 |
Mg2+ |
+ |
2 |
[NO3]– |
+ |
6 |
H2O |
Mg(NO3)2 .6H2O |
Na+ |
+ |
[SiO3]2– |
Na2SiO3 solution |
Na+ |
+ |
[OH]– |
+ |
H2O |
NaOH(aq) |
Ca2+ |
+ |
2 |
[OH]– |
Ca(OH)2(aq) |
Ba2+ |
+ |
[SO4]2– |
+ |
H2O |
BaSO4 |
Na+ |
+ |
[ClO]– |
NaOCl(aq) |
Na+ |
+ |
[CH3COO]– |
CH3COONa |
BaCl2 |
+ |
H2SO4(aq) |
+ |
H2O |
BaSO4 |
+ |
2 |
HCl |
BaCl2 |
+ |
Na2SO4 |
+ |
H2O |
BaSO4 |
+ |
2 |
NaCl |
Li+ |
+ |
Li+ |
+ |
Li+ |
+ |
[(CH3)3C]– |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com