
Interactions | Reactions | Processes
Classified as: Type 5 Lewis Acid/Base Complexation Chemistry
Type 5 complexes form when s-LUMO Lewis acids interact with hydride ions to form saline hydrides. (The reaction actually proceeds via a redox reaction between the metal and hydrogen.)
Saline hydrides Type 5 complexes may act as very strong bases (potassium hydride is one of the strongest bases available) which react with weakly acidic protons to form H2 or as hydride donor reducing agents. It turns out that for a number of subtle reasons organic chemists are more likely to regard and employ saline hydride reagents as proton abstracting bases, and inorganic chemists are more likely to regard and utilise them as reducing agents. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.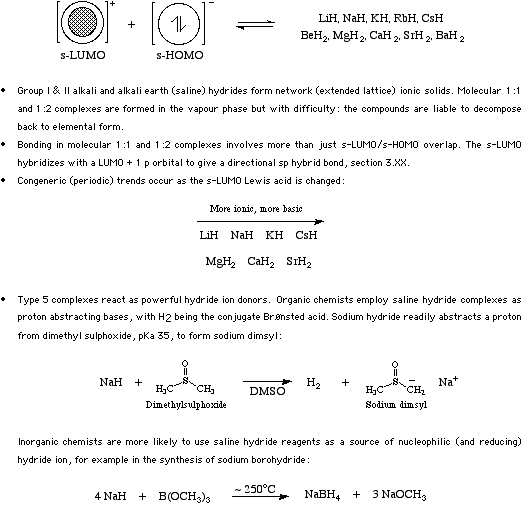
Na+ |
+ |
H– |
NaH |
+ |
Li+ |
+ |
H– |
LiH |
K+ |
+ |
H– |
KH |
Rb+ |
+ |
H– |
RbH |
Cs+ |
+ |
H– |
CsH |
Be2+ |
+ |
2 |
H– |
BeH2 |
Mg2+ |
+ |
2 |
H– |
MgH2 |
Ca2+ |
+ |
2 |
H– |
CaH2 |
Sr2+ |
+ |
2 |
H– |
SrH2 |
Ba2+ |
+ |
2 |
H– |
BaH2 |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com