
Interactions | Reactions | Processes
Classified as: Type 4 Lewis Acid/Base Complexation Chemistry
Type 4 complexes form when pi-System Lewis bases are protonated. Type 4 Lewis acid/base complexes may be very weak Brønsted acids (for example toluene, the H+/benzyl anion complex, pKa 42) or they may be strong acids like protonated ethylene (the methyl carbenium ion) pKa 15.
For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.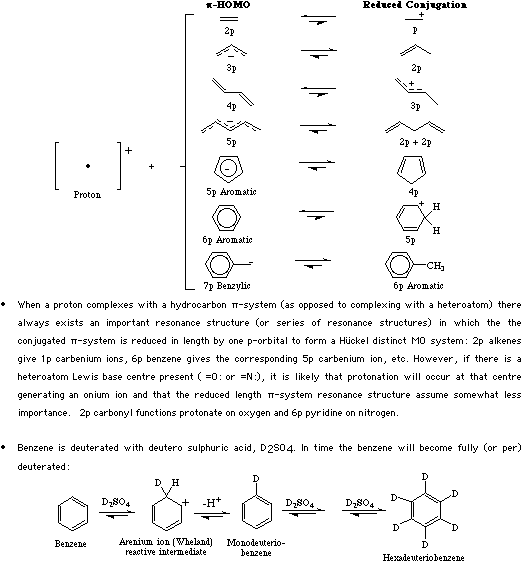
H+ |
+ |
H+ |
+ |
H+ |
+ |
H+ |
+ |
H+ |
+ |
H+ |
+ |
CH3–CH=CH2 |
H+ |
+ |
H+ |
+ |
H+ |
+ |
R+ |
H+ |
+ |
H+ |
+ |
+ |
H+ |
+ |
H+ |
+ |
[IO4]– |
HIO4 |
H2C=CH2 |
+ |
HBr |
+ |
AlBr3 |
CH3CH2Br |
+ |
H+ |
CH3–CH=CH2 |
+ |
H+ |
[CH3CH2]+ |
H2C=CH2 |
+ |
H+ |
+ |
H+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com