
Interactions | Reactions | Processes
Classified as: Type 2 Lewis Acid/Base Complexation Chemistry
Type 2 complexes form when Proton Lewis acids complex with Complex Anion Lewis bases. The resulting complexes were termed "super acids" George Olah (1994 Nobel prize) because they are such exceedingly strong proton donors: Super Acids have pKa values in the range 20 to 25.
Super acids are somewhat difficult to form as they require exotic solvents such as liquid sulfur dioxide, however, once formed they will protonate almost anything, including alkanes. For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.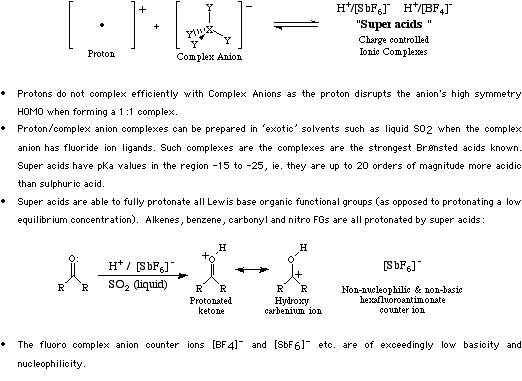
H+ |
+ |
[SbF6]– |
ROH |
+ |
H+ |
+ |
[BF4]– |
H+ [BF4]– |
SbF5 |
+ |
HSO3F |
ROH |
+ |
SO3 |
H+ |
+ |
CH4 |
[CH5]+ |
H+ |
+ |
[AlCl4]– |
AlCl3 |
+ |
HCl |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com