
Interactions | Reactions | Processes
Classified as: Type 1 Lewis Acid/Base Complexation Chemistry
Type 1 complexes form when Proton Lewis acids complex with s-HOMO Lewis bases (hydride ions, hydrogen or deuterium or tritium isotopic homologs). There are therefore of two general types of Type 1 Lewis acid/base complex: H2 and [H3]+. Both types of complex possess two electrons in a single MO, the 1s HOMO.
For more information look in the Chemogenesis webbook sections on Lewis acids and Lewis bases and the Lewis acid/base interaction matrix.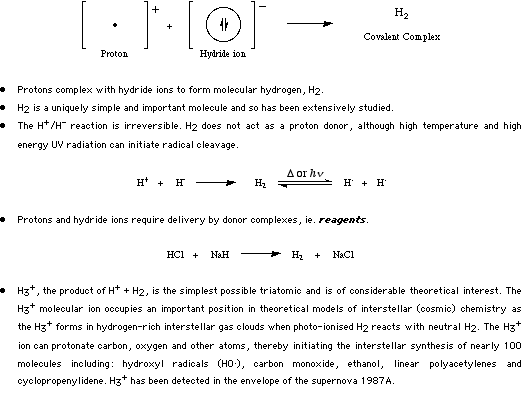
D+ |
+ |
D– |
D2 |
H+ |
+ |
H– |
H2 |
H+ |
+ |
D2 |
+ |
H+ |
+ |
He |
[HHe]+ |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com